CE Marking for Medical Devices
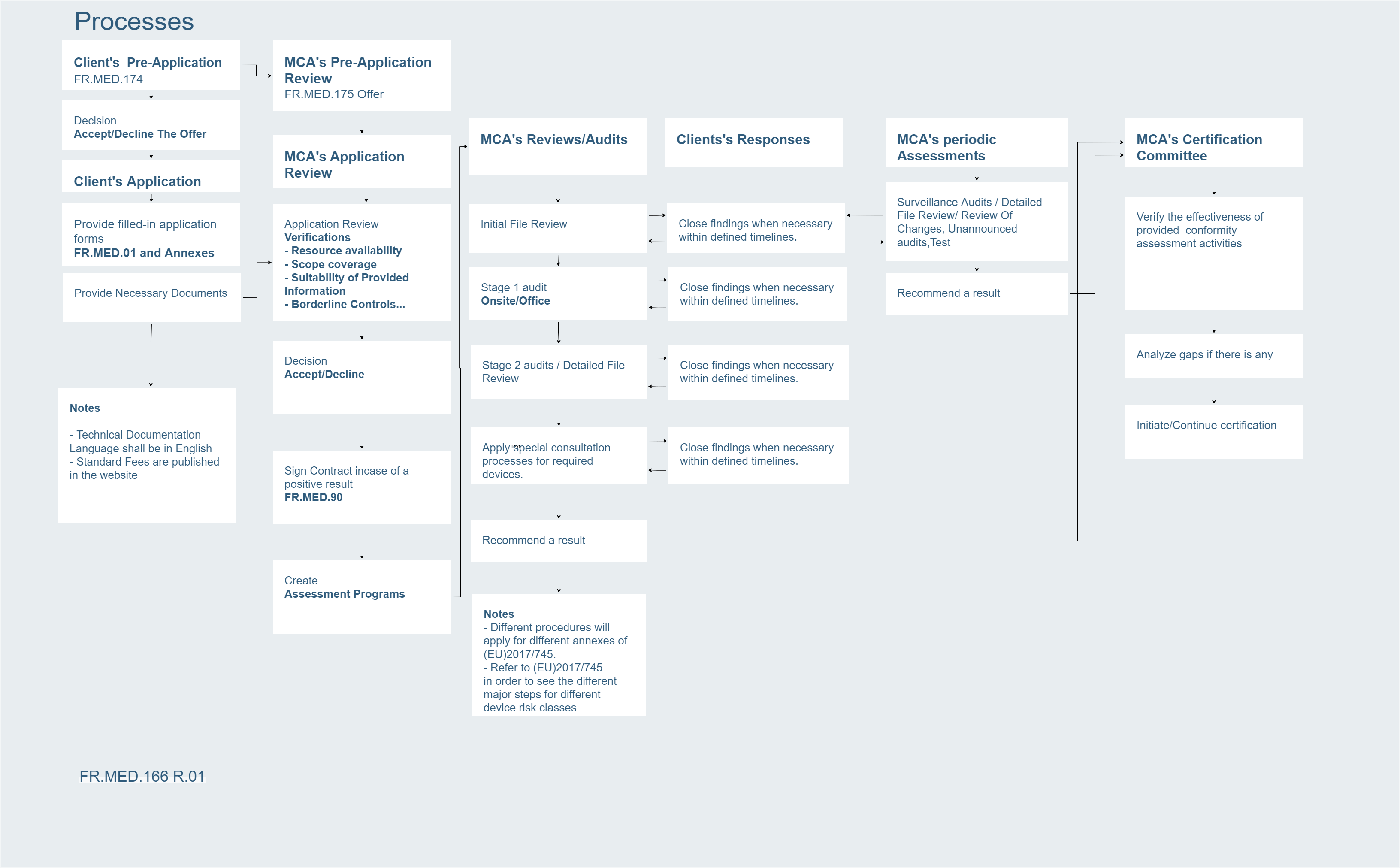
MCA initiated application for notification as a Notified Body under (EU)2017/745 .
Which services MCA provide for Medical Devices?
MCA initiated application for notification as a Notified Body under (EU)2017/745 including Annex IX Part I, Annex IX Part II and Annex XI Part A.